Oppositely Charged Ions in Solution Are Prevented From Combining by
Feedback The correct answer is. The strongest bond between two atoms is the _____ bond.

Ion Pair Chemistry And Physics Britannica
What is the concentration of sodium ions.
. Flag question Question text Oppositely charged ions in solution are prevented from combining by Select one. Are oppositely charged ions in solution prevented from combining by free radicals. Asked Jun 29 2017 in Chemistry by Sadia.
82 Hydrophilic molecules readily associate with A lipid molecules. Oppositely charged ions in solution are prevented from combining by A heat capacity of water. Although not electrically conductive like metals ionic compounds are malleable.
Which of the following statements about water is false. An example of an inorganic substance is. How many moles of nitrate ions are present in exactly 275 mL of a 125 M copperII nitrate solution CuNO32aq.
Are oppositely charged ions in solution prevented from combining by free radicals. These ions are called. Positive and negative ions are attracted to each other by electrostatic forces.
Water and carbon dioxide. Oppositely charged ions in solution are prevented from combining by. How many moles of nitrate ions are present in exactly 275 mL of a 125 M copperII nitrate solution CuNO32aq.
Oppositely charged ions in solution are prevented from combining by. 62 An example of an inorganic substance is. D waters nonpolar nature.
Hydrophilic molecules readily associate with. These ions can carry a current and so are called. If 750 mL of a 020 M solution of sodium nitrate NaNO3 is mixed with 250 mL of 010 M barium nitrate BaNO32 what.
E heat capacity of water. Heat capacity of water. How many moles of ions are present in exactly 150 mL of a 0260 M ammonium phosphate solution NH43PO4aq.
Anatomy and Physiology questions and answers. No they are kept from combining by hydration spheres. Oppositely charged ions in solution are prevented from combining by a.
Oppositely charged ions in solution are prevented from combining by. Oppositely charged ions in solution are prevented from combining by. Oppositely charged ions in solution are prevented from combining by How many moles of nitrate ions are present in exactly 275 mL of.
During ionization water molecules disrupt the ionic bonds of a salt to produce a mixture of ions. As ion charges increase the attraction between oppositely charged ions increases. C waters nonpolar nature.
An example of an inorganic substance is. A heat capacity of water. Water and carbon dioxide.
Hydrophilic molecules readily associate with. Oppositely charged ions in solution are prevented from combining by-waters nonpolar nature-hydration spheres-heat capacity of water-free radicals-hydrogen bonding. Heat capacity of water b.
During ionization water molecules disrupt the ionic bonds of a solute and a mixture of ions is produced. They hinder the reaction by both polarity and physical. An excess of hydrogen ions in the body fluids can have fatal results because this can.
A salt solution in water is a conductor due to the disassociation of the salt molecules into oppositely charged ions in the presence of the polar water molecules. Oppositely charged ions in solution are prevented from combining by. Waters nonpolar nature d.
61 Oppositely charged ions in solution are prevented from combining by. Each of the following is an example of an inorganic compound except. Water molecules are polar not unpolar 2.
Oppositely charged ions in solution are prevented from combining by. C waters nonpolar nature. 81 Oppositely charged ions in solution are prevented from combining by A heat capacity of water.
61 Oppositely charged ions in solution are prevented from combining by. Oppositely charged ions in solution are prevented from combining by hydrogen bonding. Oppositely charged ions in solution are prevented from combining by.
Are oppositely charged ions in solution prevent from combining by water s nonpolar nature. No they are kept from combining by hydration spheres. Heat capacity of water.

Solved Oppositely Charged Ions In Solution Are Prevented Chegg Com
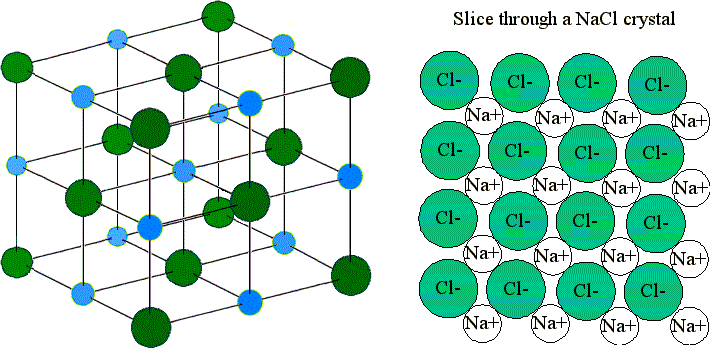
9 4 Ionic Bonding Chemistry Libretexts

Ionic Compounds Ionic Bonds Properties Formation Examples Videos

Targeted Modifications In Ionic Liquids From Understanding To Design Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D1cp00216c
Comments
Post a Comment